What Is Addition Reaction Class 11
Think of an addition reaction as two different reactants combined in a large single product that. There exist several different categories of polymerization reactions the most notable of which being step-growth polymerization chain-growth polymerization both of which fall under the category of addition polymerization and condensation polymerization.
Illustrated Glossary Of Organic Chemistry Anti Markovnikov Addition
It is a more stable.

What is addition reaction class 11. A addition reaction occurs when atoms are added to a compound containing a double or triple bond. Oxidation is defined as the addition of oxygenelectronegative element to a substance or rememoval of hydrogen electropositive element from a susbtance. It is a class of an organic addition reaction that continues in a step-wise style to create the addition product regularly in equilibrium and a water molecule later named condensation.
Redox Reactions Class 11 Notes Chemistry Chapter 8. Addition reactions occur with unsaturated compounds. An addition reaction in organic chemistry is in its simplest terms an organic reaction where two or more molecules combine to form a larger one the adduct.
The general equation for an addition reaction. Here we will see the difference between oxidation and reduction reaction. 22 cycloaddition reaction 42 cycloaddition reaction Acid-catalysed dehydration of alcohols addition reaction aldol condensation condensation recation Diel-Alder reaction Electrophilic addition reactions Electrophilic.
Addition reactions are typical of unsaturated organic compoundsie alkenes which contain a carbon-to-carbon double bond and alkynes which have a carbon-to-carbon triple bondand. Addition reactions are limited to chemical compounds that have multiple bonds such as molecules with carboncarbon double bonds alkenes or with triple bonds alkynes and compounds that have rings which are also. An electrophilic addition reaction is an addition reaction which happens because what we think of as the important molecule is attacked by an electrophile.
A photodecomposition reaction is a type of decomposition reaction in which the reactant is broken down to its constituents by absorbing energy from photons. Elimination reaction often competes with substitution reactions. NCERT Solutions For Class 11.
TextAtextB to textC Notice that C is the final product with no A or B remaining as a residue. All the atoms in the original molecules are found in the bigger one. Elimination reactions occur with saturated compounds.
Nucleophilic addition reaction of alkynes is generally catalysed by the presence of heavy metal salts such as those of mercury and barium. Reduction is defined as the memoval of oxygenelectronegative element from a substance or addition of hydrogen or electropositive element to a substance. Nothing is lost in the process.
An elimination reaction occurs when a reactant is broken up into two products. The function of mercury ions Hg2 or barium ions Ba2 is to form 7r-complex by withdrawing the electron density from the -cloudAs a result the electron density in the triple bond gets considerably reduced and therefore the nucleophilic attack is. Reaction of butene with hydrobromic acid.
Two possible mechanisms are available for this elimination reaction E1 and E2 mechanisms. In this reaction a substrate typically an alkyl halide eliminates one equivalent unit of acid to form an alkene. It is also a fact that these are the balanced chemical reactions.
But only chemical compounds containing multiple bond character can undergo an addition reaction as a double or triple bond is usually broken to form the required single bonds. NCERT Solutions For Class 11 Physics. Addition reaction any of a class of chemical reactions in which an atom or group of atoms is added to a molecule.
The reaction could otherwise include the functional groups of the molecule. It is observed in this reaction that the hydroxyl group attaches itself to the carbon with more carbon-carbon bonds whereas the hydrogen atom attaches itself to the other carbon in the. This reaction proceeds via the formation of a carbocation.
Chemistry Class 11 Organic Chemistry - Some Basic Principles and Techniques Tagged With. Reaction of propene with HI Application of Markovnikovs rule in addition reactions of aromatic alkene-Reaction of propyne with HBr-Reasoning behind Markovnikovs Rule. In the simplest of terms of organic chemistry we can say that an addition reaction is a chemical reaction wherein two or more reactants come together to form a larger single product.
What is an Addition Reaction. An addition reaction is a reaction in which two molecules join together to make a bigger one. Let us consider the addition reaction wherein an alkene reacts with water to give rise to an alcohol.
Electrophilic addition of acidic hydrogen ion or proton on alkene results in formation of carbocation. An example of a photo decomposition reaction is the decomposition into dioxygen and an oxygen radical as represented by the chemical equation provided below. A Chemical reaction is the process in which one or more substances react and hence as a result then converted to one or more different substances.
 Addition Reaction Definition Examples And Mechanism
Addition Reaction Definition Examples And Mechanism
 Addition Reaction Of Alkenes For Class 11 Chemistry 11th Chemistry Chemistry Physics Research
Addition Reaction Of Alkenes For Class 11 Chemistry 11th Chemistry Chemistry Physics Research
 Markovnikov S Rule Definition Explanation Of Mechanism With Examples
Markovnikov S Rule Definition Explanation Of Mechanism With Examples
 Reactions Of Dienes 1 2 And 1 4 Addition Master Organic Chemistry
Reactions Of Dienes 1 2 And 1 4 Addition Master Organic Chemistry
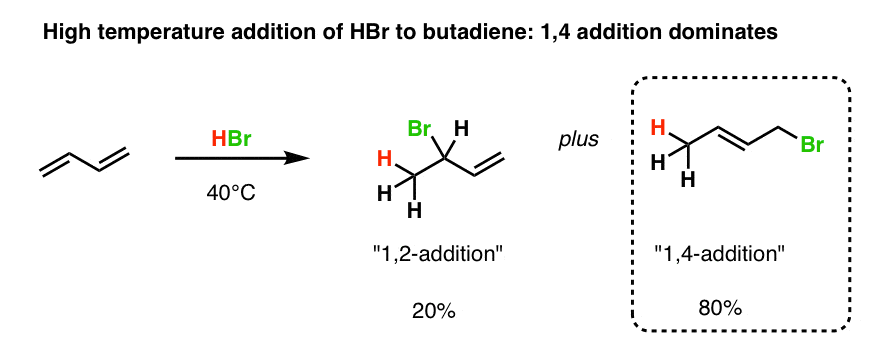 Reactions Of Dienes 1 2 And 1 4 Addition Master Organic Chemistry
Reactions Of Dienes 1 2 And 1 4 Addition Master Organic Chemistry
 Markovnikov S Rule Definition Explanation Of Mechanism With Examples
Markovnikov S Rule Definition Explanation Of Mechanism With Examples
 Alkadiene An Overview Sciencedirect Topics
Alkadiene An Overview Sciencedirect Topics
 Difference Between Addition And Substitution Reactions Comparison Summary Chemistry Notes Chemistry Lessons Reactions
Difference Between Addition And Substitution Reactions Comparison Summary Chemistry Notes Chemistry Lessons Reactions
 Addition Reaction Definition Examples And Mechanism
Addition Reaction Definition Examples And Mechanism
 Addition Reaction Definition Examples And Mechanism
Addition Reaction Definition Examples And Mechanism
 Reactions Of Dienes 1 2 And 1 4 Addition Master Organic Chemistry
Reactions Of Dienes 1 2 And 1 4 Addition Master Organic Chemistry
 Addition Reaction Definition Examples And Mechanism
Addition Reaction Definition Examples And Mechanism
Illustrated Glossary Of Organic Chemistry Markovnikov S Rule Markovnikov Addition
 Best Chemistry Blogs Reactions Of Aldehyde Ketone With Hcn Nahso3 Ald Chemistry Aldol Condensation Chemistry Free
Best Chemistry Blogs Reactions Of Aldehyde Ketone With Hcn Nahso3 Ald Chemistry Aldol Condensation Chemistry Free
 Electrophilic Addition Reactions Of Hydrogen Halides Hx To Alkenes Hcl Science Education Organic Chemistry Organic Chemistry Tutor
Electrophilic Addition Reactions Of Hydrogen Halides Hx To Alkenes Hcl Science Education Organic Chemistry Organic Chemistry Tutor
 Nucleophilic Addition Reaction Aldehydes Are More Reactive Than Ketones Electron Donating Groups Ketones Hydrogen Atom
Nucleophilic Addition Reaction Aldehydes Are More Reactive Than Ketones Electron Donating Groups Ketones Hydrogen Atom
 Addition Reaction Definition Examples And Mechanism
Addition Reaction Definition Examples And Mechanism
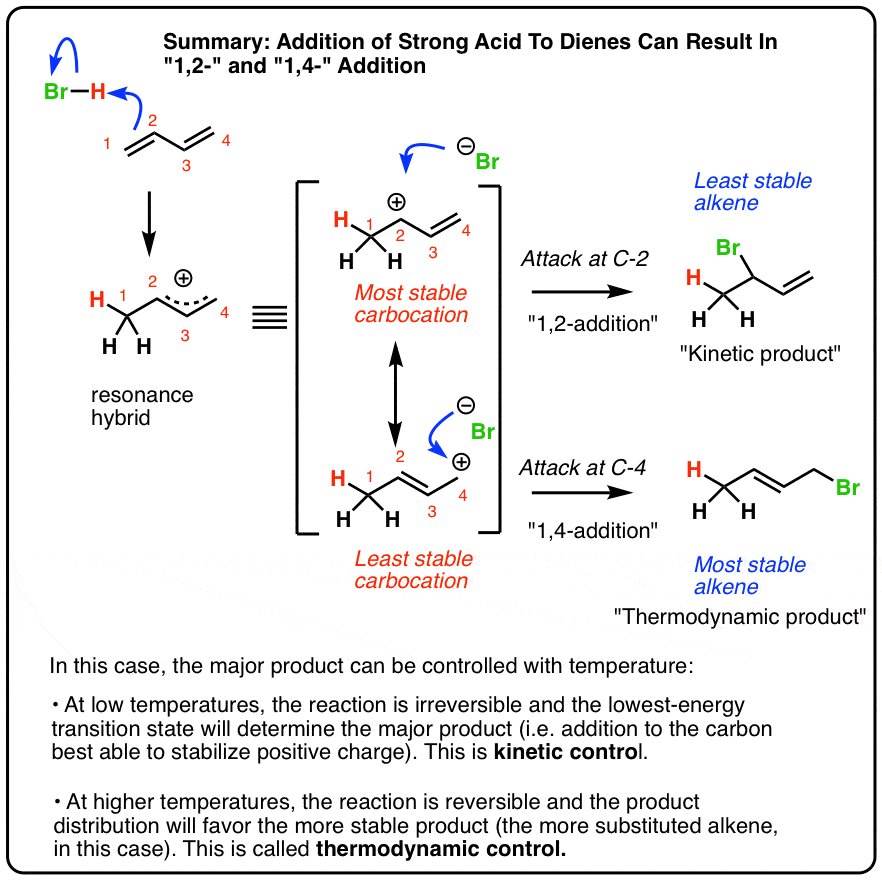 Reactions Of Dienes 1 2 And 1 4 Addition Master Organic Chemistry
Reactions Of Dienes 1 2 And 1 4 Addition Master Organic Chemistry
 Addition Reaction Addition Reactions Of Alkenes Markovnikov Rule Organic Chem Organic Chemistry Reactions
Addition Reaction Addition Reactions Of Alkenes Markovnikov Rule Organic Chem Organic Chemistry Reactions